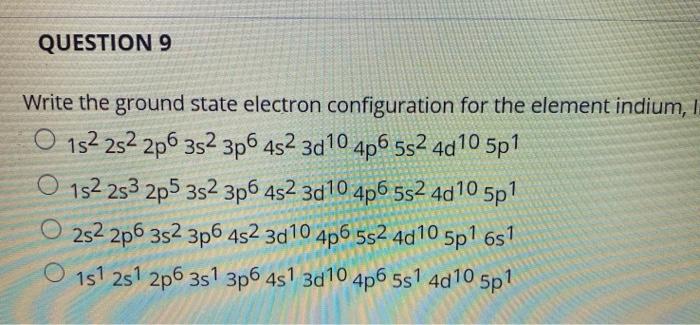
Write the Complete Ground-state Electron Configuration of Indium.
Possible oxidation states are 3. Rn 5f 14 6d 10 7s 2 7p 6.

Indium In Electron Configuration And Orbital Diagram
This give us the correct configuration of.

. For the Cu2 ion we remove a total of two electrons one from the 4s1 and one form the 3d10 leaving us with. Cadmium indium tin. Its electron configuration is.
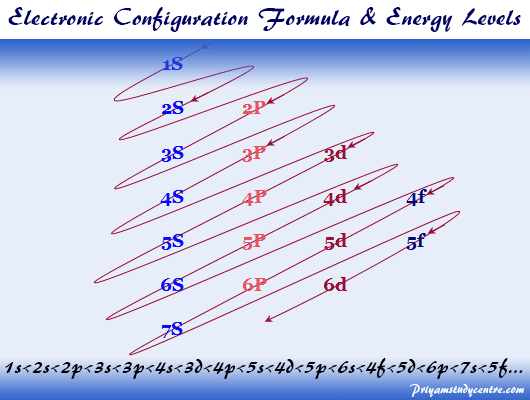
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. Write the complete ground-state electron configuration of aluminum. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6.
Melting point C 15663. List the known quantities and plan the problem. Indium Complete Electron Configuration.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 1. How is vsepr used to classify molecules. The ground state electron configuration of ground state gaseous neutral indium is Kr.
How do I determine the molecular shape of a molecule. Nevertheless check the complete configuration and other interesting facts about Indium that most people dont know. Known carbon atomic number Z 6 Use the order of the fill diagram to draw an orbital fill diagram with a total of six electrons.
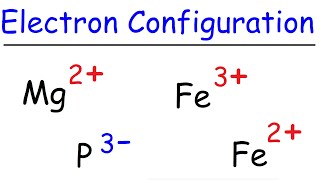
Examine the electron configurations of several atoms to determine which atom has the fewest unpaired electrons. Solution for Write the complete ground-state electron configuration of Fe2. Element Answer Ar3d10 451 Cu 5.
Write the electron configuration. Write the complete ground state electron configuration for each of the following ions. Boiling point C 2080.
For example 152 22 2p should be entered as 152 2s22p6. Element Answer Si3 13 Write the noble gas core abbreviated electron configuration for each of the following elements and ions. Electron configuration of Indium is Kr 4d10 5s2 5p1.
Write the condensed noble-gas electron configuration of indium. A step-by-step description of how to write the electron configuration for Indium InIn order to write the In electron configuration we first need to know t. Full electron configuration of indium.
We first need to find. Enter answers with a space between orbitals. This electron configuration shows that the last shell of a bromine atom has an unpaired electron.
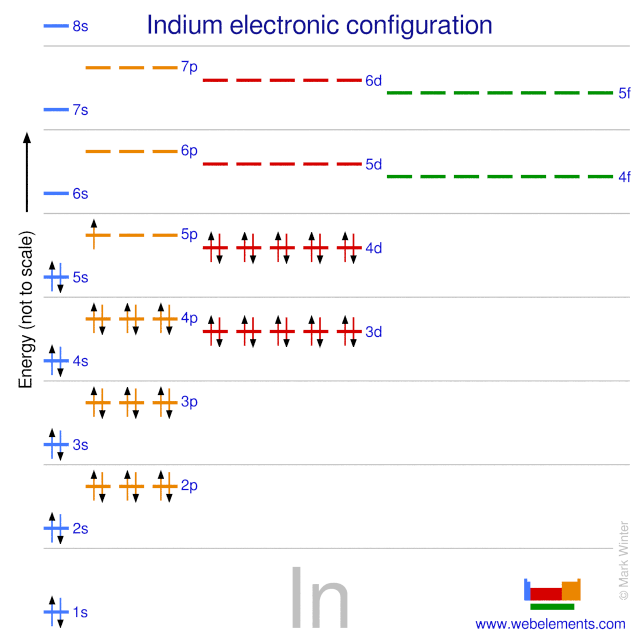
Indium atoms have 49 electrons and the shell structure is 2818183. In the case of Indium the abbreviated electron configuration is Kr 4d10 5s2 5p1. Density gcm 3 731.
From the electrons in an atom to the differing orbitals and hybridization the ground state electron configuration sheds light on many different atomic. For the Cu ion we remove one electron from 4s1 leaving us with. Write the complete ground-state electron configuration of arsenic Ground state electron configurations are the foundation for understanding molecular bonding properties and structures.
Then the correct electron configuration of bromineBr in the ground state will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p x 2 4p y 2 4p z 1. Alternatively write the symbol for the noble gas before an element radon in this case and just add the extra information. 5p 1 and the term symbol is 2 P 12.
What is the lewis structure for hcn. What is the lewis structure for co2. Chemistry questions and answers.
Kr 5s2 4d10 5p1. Write the complete ground-state electron configuration for Al 3 1s²2s²2p⁶3s²3p⁶4s². Each sub-orbital can have a maximum of two electrons.
1s2 2s2 2p6 3s2 3p6 4 s2 3 d10 4 p6 5 s2 4 d10 5 p1. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p1. Write the complete ground-state.
What oxidation state would be reasonable for indium on this basis. To write the configuration for the Silver and the Silver ion first we need to write the electron configuration for just Silver Ag. The configuration notation provides an easy way for scientists to write and communicate how.
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p1. Draw the orbital fill diagram for carbon and write its electronic configuration. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 1 Back to key information about the elementBack to.
So in this case the valency of bromine is 1. Follow the rule of Hundâ. Keep in mind electron configurations are most stable when they are filled or half-filled.
Indium is a chemical element with atomic number 49 which means there are 49 protons and 49 electrons in the atomic structureThe chemical symbol for Indium is In. The ground state electron configuration for Indium In is. Write the complete ground-state electron configuration of indium 1 1 2 3 4 5 6 s pdf 2 3 04 05 D.
Electron Configuration and Oxidation States of Indium.
Electron Configuration Periodic Table Elements Chemistry

Electron Configuration For Boron B

How To Write The Electron Configuration For Indium In Youtube
Electron Configuration Of Ions Mg2 P3 Fe2 Fe3 Youtube
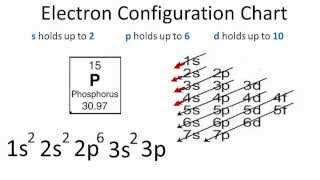
Electron Configuration For Phosphorus P

Pin On Chemistry Periodic Table
Webelements Periodic Table Indium Properties Of Free Atoms

Electronic Configuration Indium Youtube

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes

Different Strategies For Determining Electron Configurations Of Download Scientific Diagram

3 1 Electron Configurations Problems Chemistry Libretexts

Indium In Electron Configuration And Orbital Diagram

Fluorine F Electron Configuration And Orbital Diagram

Fluorine F Electron Configuration And Orbital Diagram

How To Write The Electron Configuration For Indium In Youtube
Solved Question 9 Write The Ground State Electron Chegg Com

Write The Electron Configuration Of Silver Ag And Ag Youtube

Electron Configuration Of Indium In Lesson Youtube

Electron Configurations For The Third And Fourth Periods Video Khan Academy
Comments
Post a Comment